Fluorine Has a Higher Ionization Energy Than Oxygen Because
In the nineteenth century doctors and scientists. The ionization energy associated with removal of the first electron is most commonly used.

Solved Periodicity True False Activity Group Members Your Chegg Com
FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation.
. Carbon graphite nitrogen. However because fluorine is such a small atom you are putting the new electron into a region of space already crowded with electrons and there is a significant amount of repulsion. It takes far less energy however to remove an electron from a lithium atom which has three protons in its nucleus.
Metals usually have higher melting and boiling points than non-metals so lets examine the melting points of period 2 elements. It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compoundsOxygen is Earths most abundant element and after hydrogen and helium it is the third-most abundant element in the. 3272 -210 -219 -187 -2486.
The liquid phase has a higher internal energy than the solid phase. In addition to H possible electron-pair acceptors Lewis acids include neutral molecules such as BF 3 and high oxidation state metal ions such as Ag 2 Fe 3 and Mn 7. This means energy must be supplied to a solid in order to melt it and energy is released from a liquid when it freezes because the molecules in the liquid experience weaker intermolecular forces and so have a higher potential energy a kind of bond-dissociation energy for intermolecular forces.
All of the obtained XPS spectra were analyzed by CasaXPS software and calibrated with a hydrocarbon C 1s signal at 2846 eV. The higher the shielding effect the lower the ionization energy. A Lewis base or electron-pair donor is a molecule with one or more high-energy lone pairs of electrons which can be shared with a low-energy vacant orbital in an acceptor molecule to form an adduct.
The nth ionization energy refers to the amount of energy required to remove an electron from the species with a charge of n-1. The incoming electron is going to be closer to the nucleus in fluorine than in any other of these elements so you would expect a high value of electron affinity. XPS was performed with a pass energy of 15 kV and high resolution scans with a step size of 005 eV were collected after a survey scan with a step size of 10 eV for lithium 1s carbon 1s oxygen 1s nitrogen 1s fluorine 1s and cobalt 2p regions.
Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. Oxygen is the chemical element with the symbol O and atomic number 8. Ionization potential refers to the energy required as input to an atom to remove the first valence electron ie a measure of how tightly bound electrons are to the atom and the electron.
Showing the increasing trend of ionization energy in KJmol exception in case of Boron from left to right in the periodic table8 Li 520. Name of Element. Chlorine has been used for applications such as the deactivation of pathogens in drinking water swimming pool water and wastewater for the disinfection of household areas and for textile bleaching for more than two hundred years.
Lig Li g e-H o 5723 kJmol. The major role of fluorine-containing gases in increasing the atomic oxygen density is through increased number density of electrons or the energy of electrons. Heg He g e-H o 23723 kJmol.
X X 2 e. X 2 X 3. X X e.
The elements on the right nitrogen oxygen fluorine and neon all have low. When chlorine was discovered we did not now that disease was caused by microorganisms. Melting Point C 180.
The first ionization energy for helium is slightly less than twice the ionization energy for hydrogen because each electron in helium feels the attractive force of two protons instead of one. Though the increased etching rate with the addition of fluorine-containing gases is through the enhancement of oxygen atom density fluorine can also take part in the reactions to generate polymer radical sites through.

Unit 1 Atomic Theory And Structure

Solved Which Of The Following Statements Is Correct More Chegg Com

Periodic Trends In Ionization Energy Ck 12 Foundation
Why Is The 1st Ionization Energy Of Ne Greater Than F Quora
The Second Ionization Energy Is Always Greater Than The First Ionization Energy How Is The Second Ionization Of O More Than N But The First Ionization Energy Of N Is More Than

If Oxygen Has Low Ionization Energy Because One Of The P Orbitals Is Filled With Two Electrons That Repeal Each Other Then Why Doesn T Fluorine Have Lower Ionization Energy Than Oxygen
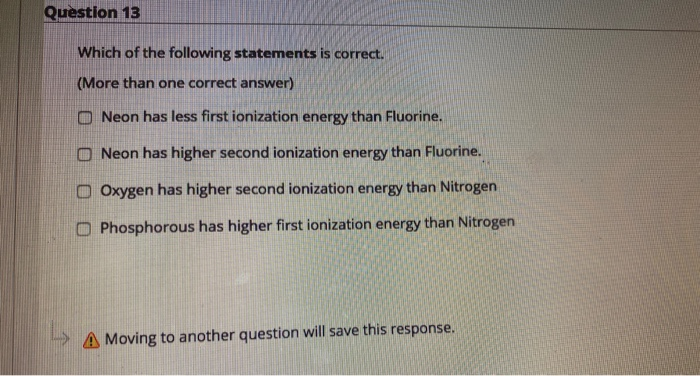
Solved Question 13 Which Of The Following Statements Is Chegg Com

Periodic Trends Worksheet Answers Page 1 1 Rank The Following
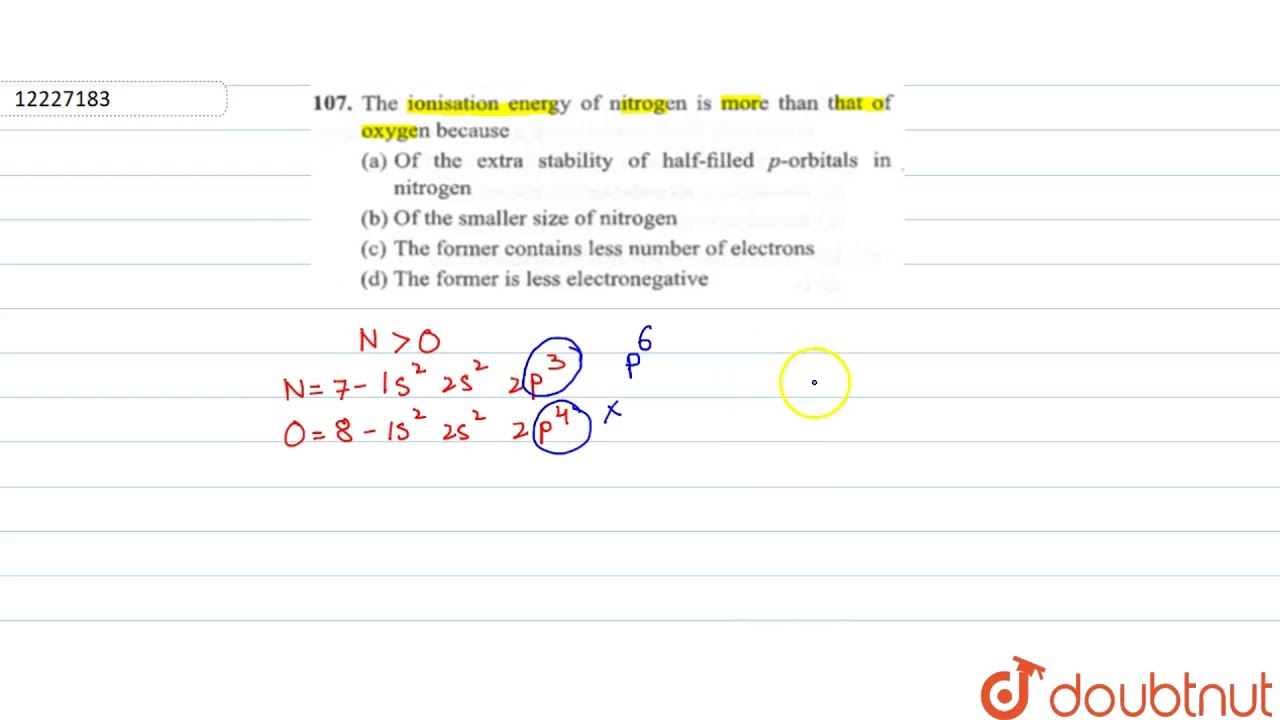
The Ionisation Energy Of Nitrogen Is More Than That Of Oxygen Because Youtube
Why Doesnt Fluorine Have Lower Ionization Energy Than Oxygen Quora
Why Does Neon Have Higher Ionisation Energy Than Oxygen Quora
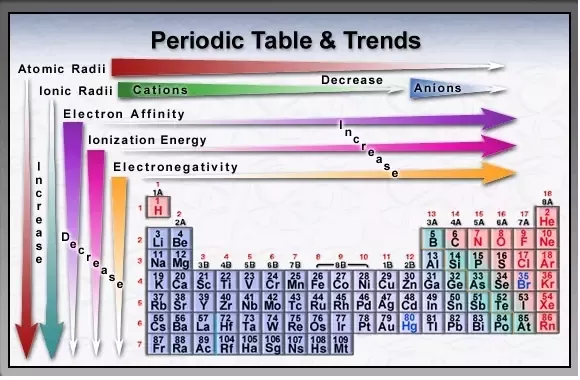
Why Are The Ionization Energies Of Boron B Oxygen O Aluminium Al And Sulphur S Less Than Than Those Of Beryllium Be Nitrogen N Magnesium Mg And Phosphorus P Respectively Quora

Periodic Trends If Fluorine Has A Lower Electron Affinity Than Chlorine Why Does It Have A Higher Ionization Energy Chemistry Stack Exchange

Fluorine Has Lower Electron Affinity Than Chlorine Because Of Youtube

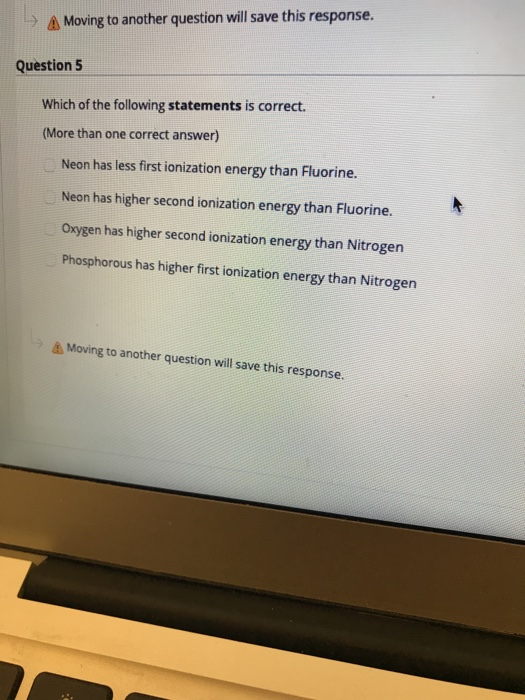
Solved A Moving To Another Question Will Save This Chegg Com
Why Does Nitrogen Have A Higher Ionization Energy Than Carbon Quora

Solved Question 2 1 Point Saved Which Of The Following Chegg Com

Solved Identify The Reasons Why Oxygen Has A Lower First Chegg Com

In Questions 2 4 You May Use Radius Comparisons As Gi Itprospt
Comments
Post a Comment